What is the relationship between pressure and gas volume? and how does that affect freedivers
The relationship between pressure and gas volume is described by Boyle's Law, which states that at constant temperature, the pressure of a given amount of gas is inversely proportional to its volume
In mathematical terms, Boyle's Law can be expressed as:
 P⋅V=constant
P⋅V=constant
where:
- P is the pressure of the gas
- V is the volume of the gas
This means that if you have a sample of gas at a constant temperature, doubling the pressure will result in the volume being halved, and vice versa. In other words, when the volume of a gas decreases, its pressure increases, and when the volume increases, the pressure decreases, as long as the temperature remains constant.
It's important to note that Boyle's Law is applicable under the assumption of constant temperature. If the temperature changes, other gas laws like Charles' Law or the combined gas law may need to be considered.
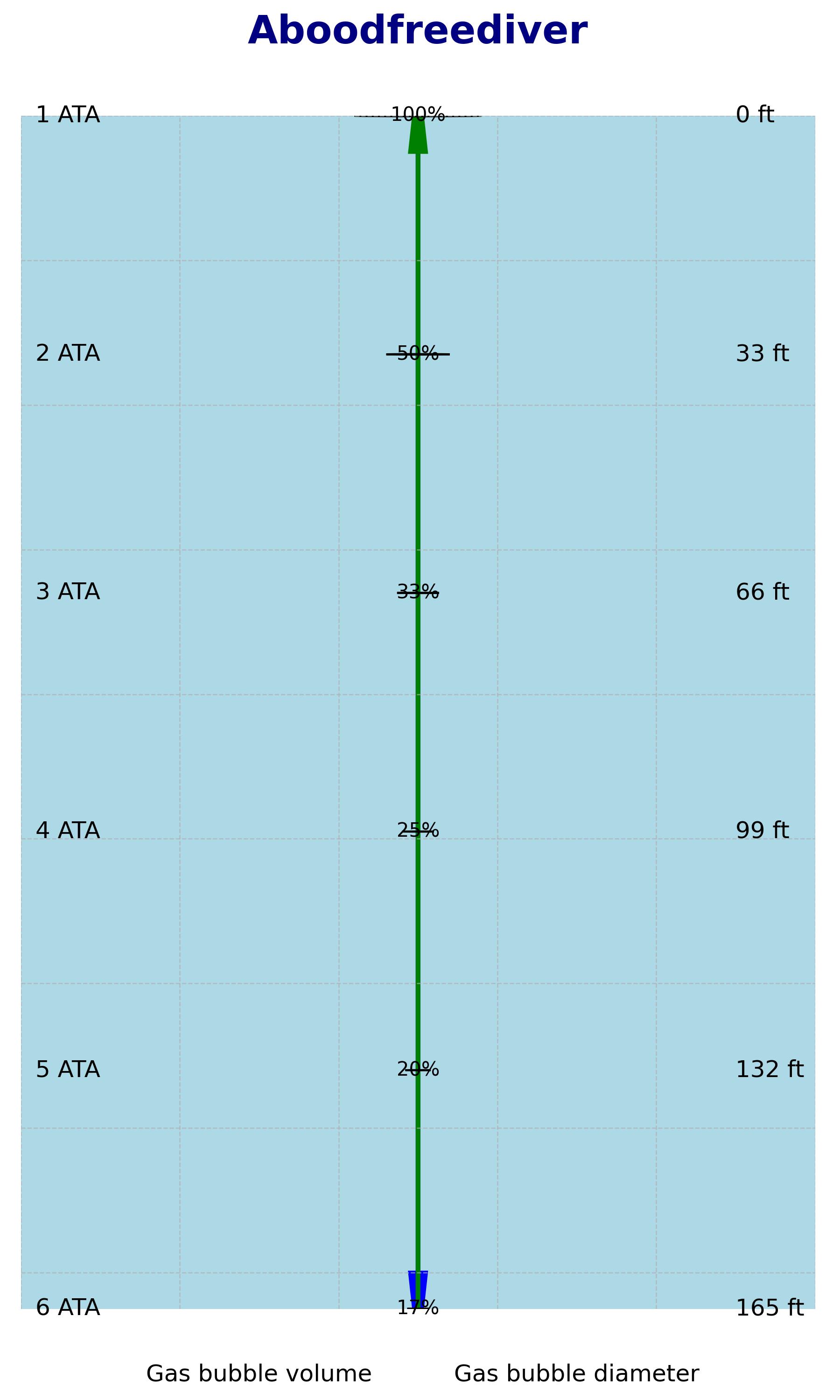
Boyle's Law has implications for freedivers, who engage in breath-holding and underwater diving without the use of breathing apparatus. As freedivers descend underwater, the pressure increases, and this affects the volume of air in their lungs.
According to Boyle's Law, as the pressure increases, the volume of a gas (in this case, the air in the lungs) decreases. Conversely, as freedivers ascend to the surface, the pressure decreases, and the volume of air in their lungs expands.
Freedivers need to be aware of these changes in pressure because they can impact lung volume, buoyancy, and the risk of barotrauma. Barotrauma refers to physical damage that can occur when there is a pressure difference between the inside and outside of the body. In the context of freediving, barotrauma can affect the ears, sinuses, and lungs.
